陳銘洲教授
新n-型多併環噻吩材料之開發中,蒸鍍型材料已開發具0.3 (AFM發表)與~0.43 cm2/Vs電性之新衍生物 (AEM 發表),於國內開發之n-型新材料中亦名列前茅,近期更大的突破是新開發之醌型多併環材料,於空氣下之元件效能可達> 0.1 cm2/Vs 電性!目前已開發出多個此新型材料,溶液製程之可達電性~ 0.1~0.5 cm2/Vs。利用本實驗室所開發之兩個最高電性的可溶性材料,溶液製程製備之ambipolar元件,p-型與n-型電性分別可達 0.2 與0.7 cm2/Vs!
新開發之併環噻吩,除可應用於 OTFT 之外,亦可應用於 OPV/2PA/DSSC等有機光電領域。目前我們開發的小分子已有4.0% 之光電轉換效能,開發中的高分子已具~7% PCE(聚合條件與元件優化中)。我們亦已開發具相當高之雙光子吸收(2PA)之多併環噻吩材料 (~3000 GM)。本實驗所開發之多併環染敏材料之DSSC光電轉換效能已達 10.1%(已於JACS發表)-為目前台灣有機染敏之最高光電轉換效能記錄。目前,我們已改良並提升此一電性紀錄11.2%(投稿中)。另外我們亦開發多個新有機染敏材料具8.5~10.2%之高光電轉換效能。
整體而言,本實驗室這幾年致力於有機多併環噻吩材料之開發,我們已可以運用所開發出來的one-pot [X+1+Y]的合成方法更快製備出更多之新多併環材料,可用於OTFT、OPV、2PA 與DSSC之多個有機光電領域的研究! 在此特別感謝科技部鼎力支持!
另外本實驗室在燃料電池的離子交換膜(DMFC/PEM)的研發上亦有文章之發表。本實驗室所開發的鹼性陰離子交換膜(AEM)之導電度已達0.1 S/cm,為台灣所開發之陰離子交換膜材之最高陰離子傳導度記錄。
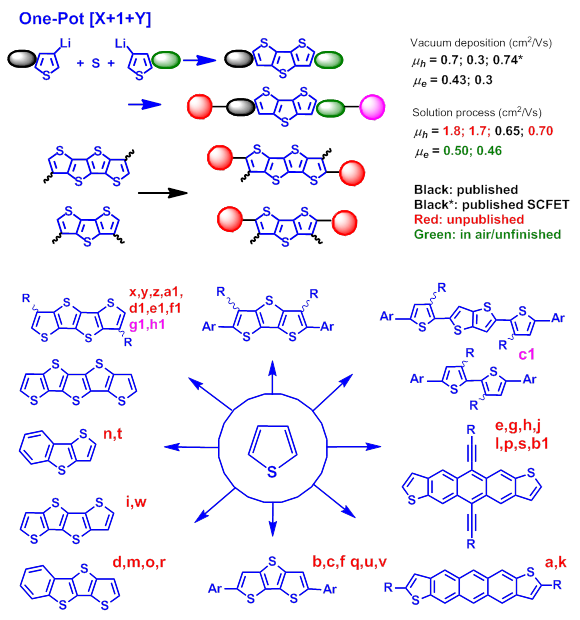
Figure 1. a. J. Mater. Chem. 2008, 1029. b. Chem. Commun. 2009, 1846. c. Org. Electronics 2010, 801. d. Chem. Mater. 2010, 5031. e. Org. Electronics 2010, 1363. f. Langmuir 2010,13353. g. Org. Electronics 2010, 1947. h. Synlett 2011, 2151. i. Adv. Funct. Mater. 2012, 48. j. Physica Status Solidi-Rapid Research Letters, 2012. 71. k. J. Phy. Chem. C. 2012, 1225. l. Org. Electronics 2012, 1881. m. ACS Appl. Mater. & Interfaces 2012, 4, 6992. n. Chemistry - A European Journal 2013, 3721. o. Adv. Funct. Mater. 2013, 3850. p. Chemphyschem, 2013, 2772. q. Surface Science, 2013, 155. r. Adv. Funct. Mater. 2014, 2057. s. J. Phys. Chem. C 2014, 9958. t. J. Mater. Chem. C. 2014, 7599. u. J. Mater. Chem. C. 2014, 8892. v. Polymers, 2014, 2645. w. Adv. Elect. Mater. 2015, 1500098. x. J. Am. Chem. Soc. 2015, 4414. y. RSC Adv. 2015, 54003. z. Chem. Asian J. 2015, 1640. a1. J. Mater. Chem. C. 2015, 8932. b1. Dyes and Pigments 2016, 126, 261. c1. Submitted to JACS. d1. Dyes and Pigments 2016, 133, 65. e1. ACS Appl. Mater. & Interfaces 2016, 15267. f1. Dyes and Pigments 2016, 280. g1. ACS Appl. Mater. & Interfaces 2016, 8, 15267. h1. Submitted to Nano Energy.
Organic field-effect transistors (OFETs) have attracted intensive attention for potential applications in flexible displays, low-cost electronic papers, RFID components, and smart textiles, as well as smart memory/sensor elements in the automotive and transportation industries.
During the past recent years, we have developed a simple [X+1+Y] one-pot synthetic route for DTT (dithenothiopehene), TTA (tetrathienoacene), and PTA (pentathieno- thiophene) syntheses. A couple new derivatives based on these fused-thiophenes have been developed. In 2014, we have reported stable soluble benzothienodithiophene (BTDT)-based materials with 0.65 cm2/Vs mobility via solution process (in AFM). New developed benzothienothiophene (BTT) showed 0.34 cm2/Vs mobility and asymmetric DTTs exhibited single crystal mobility of 0.7 cm2/V.
For p-types, we have developed two new soluble small molesules which exhibited mobilities up to 1.7 and 1.8 cm2/Vs via solution process recently and a couple new p-type small molecules exhibited 0.1~ 0.7 cm2/Vs mobilities. For n-types, new TTAs exhibited mobility up to 0.43 cm2/Vs (vacuum deposition) had been published (AEM 2015). Soluble TTA-based materials have exhibited mobilities up to 0.8 cm2/Vs via solution shearing (Chem Euro J. submitted)
Markedly, these new developed soluble fused-thiophenes can also be applied in OPV and 2PA. We have published three OPV papers. For example, our TTA-based OPV small molecules has exhibited 4% of PCE. Furthermore, we have developed new OPV polymer with ~ 7% PCE (unfinished). For 2PA, we also published three papers of fused-thiophene based small molecules with 2500~3000 GM.
Finally, the newly developed fused-thiophenes offer good building blocks for new DSSCs. Our new developed organic dyes have exhibited PCE upto 10.1%, which is the currently best PCE record in Taiwan, just published in JACS 2015. Recently, we have modified our best dye and 11.2% PCE has been achieved (paper submitted). In addition, a couple of new organic dyes based on our new developed core with 8.5~10.2% PCE have been obtained.